-KMU Office of Research and Development-
Chia-Hao Kuo , Jo-Yun Shih , Yi-Hsiung Lin , Chien-Wei Chang , Shih-Jie Jhuo , Tzu-Chieh Lin , Tien-Chi Huang , I Hsin Liu , Pei-Heng Kao , En-Ying Lin , Pin-Chieh Huang , Xin-Hui Chen , Chao-Yi Chen , Yu-Chen Feng , Ying-Xuan Zheng , Min-Huei Lin , Guan-Lin Chen , Po-Chao Hsu , Chien-Hung Lee , Chun-Chieh Wu , Hsiang-Chun Lee , Bin-Nan Wu , Shien-Fong Lin , Wen-Ter Lai , Wei-Chung Tsai*
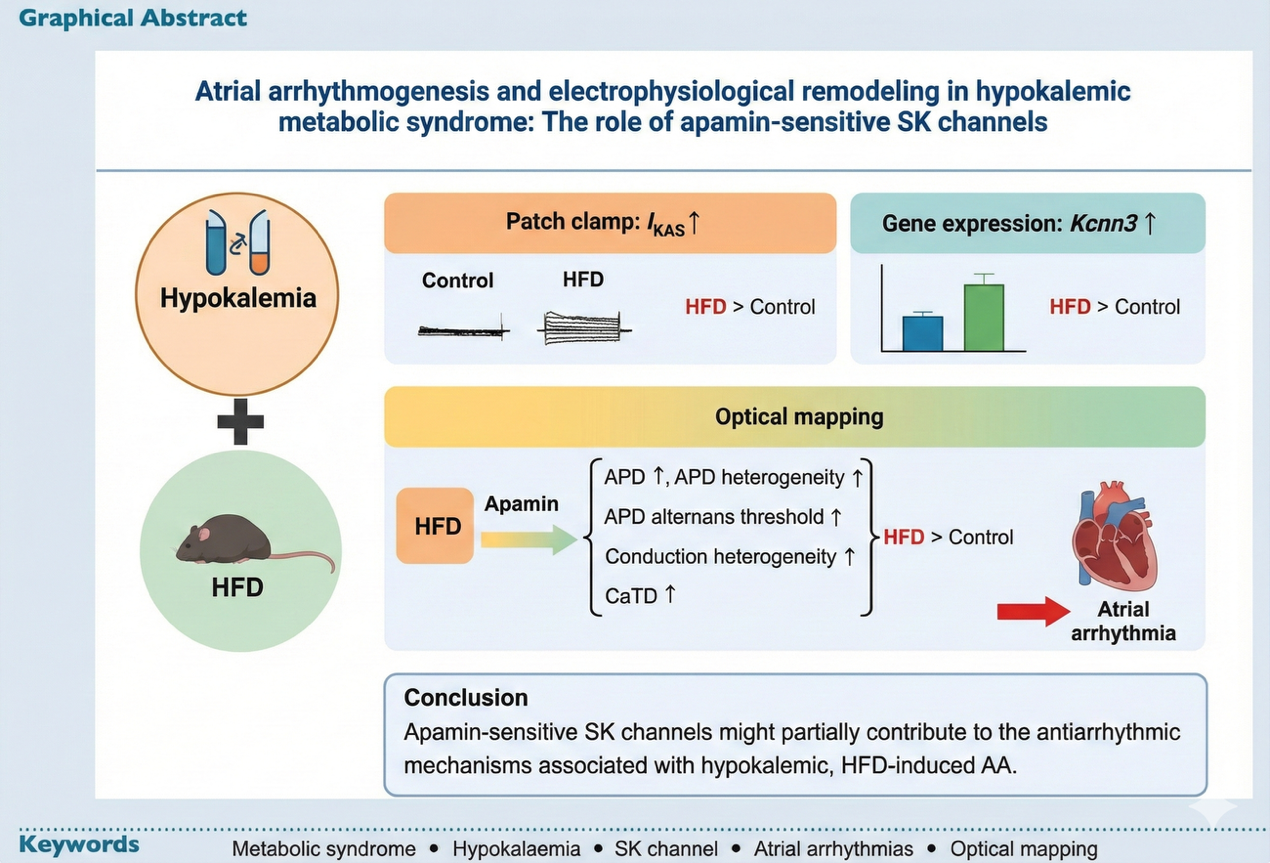
Figure 1. The visual abstract of our research: atrial arrhythmogenesis and electrophysiological remodeling in hypokalemic metabolic syndrome: the role of apamin-sensitive SK channels.
This study presents a major translational breakthrough, published in Europace, that investigates the complex mechanisms driving atrial arrhythmias (AA) in patients with both Metabolic Syndrome (MetS) and hypokalemia. Hypokalemia is a frequent comorbidity in MetS patients, often resulting from diuretic use or diet, which can increase the risk of arrhythmias.
The core aim was to clarify the role of apamin-sensitive small-conductance calcium-activated potassium (SK) channels in this specific, high-risk patient group. SK channels are calcium-sensitive potassium channels primarily expressed in the atria and typically modulate action potential duration (APD) and intracellular calcium homeostasis.
- Importance and Breakthrough Findings
The central discovery of this research challenges conventional views on SK channel function: SK channels appear to exert an antiarrhythmic, protective effect in the hypokalemic MetS heart.
- SK Channel Upregulation: Using high-fat diet (HFD) mice to mimic MetS, the researchers found a significant upregulation of the apamin-sensitive potassium current and increased expression of the gene in atrial myocytes under hypokalemic conditions.
- Increased Arrhythmia Risk Upon Blockade: The critical finding was the effect of blocking this upregulated channel using the selective blocker, apamin. When apamin was applied, the electrophysiological stability of the HFD mice hearts drastically deteriorated, suggesting the SK channels were helping to maintain rhythm.
- Electrophysiological Instability: Blocking SK channels with apamin significantly increased the inducibility of atrial arrhythmias (AA) in HFD mice (where AA was successfully induced, inducibility rose from 20% to 100%). This blockade also led to significant increases in parameters associated with instability:
-
-
- APD Heterogeneity: The variability of electrical recovery across the atrium increased.
- APD Alternans Threshold: The threshold for cardiac alternans—a periodic beat-to-beat oscillation in electrical activity linked to arrhythmias—was significantly elevated in HFD mice after apamin application, indicating a proarrhythmic effect of the blockade.
- Conduction Heterogeneity: The standard deviation of conduction angles was significantly higher in HFD mice post-apamin, indicating disorganized electrical spread.
-
In summary, this breakthrough reveals a context-dependent function of SK channels. Although SK channels have previously been implicated in promoting atrial fibrillation in HFD models generally, they display antiarrhythmic properties when hypokalemia is concurrent.
- Future Impact and Clinical Implications
These findings carry direct and critical clinical consequences.
- Clinical Practice and Safety: The research underscores the importance of evaluating potential risks associated with using SK channel blockers in patients who have hypokalemic MetS. Since MetS patients are frequently treated with diuretics that can cause hypokalemia, blocking the now-protective SK channel could induce atrial arrhythmia. The study strongly advises that plasma potassium levels should be carefully monitored, and hypokalemia should be avoided when MetS patients require SK channel blocker treatment.
- Academic and Drug Development: The complex regulatory mechanism observed—where HFD and hypokalemia interact to modulate SK channel activity—paves the way for future academic investigations.
- Mechanistic Research: Further studies are needed to confirm the regulatory mechanisms, hypothesizing that hypokalemia and HFD induce differential activation of protein kinases like CKII, CaMKII, or PP2A, thereby altering calcium sensitivity and the distribution of SK channels in the cell membrane.
- Targeted Therapy: Clarifying these mechanisms will be essential for developing new, safer antiarrhythmic drugs that account for the patient’s metabolic and electrolyte status, potentially avoiding the proarrhythmic effects seen when SK channels are blocked during concurrent low potassium.
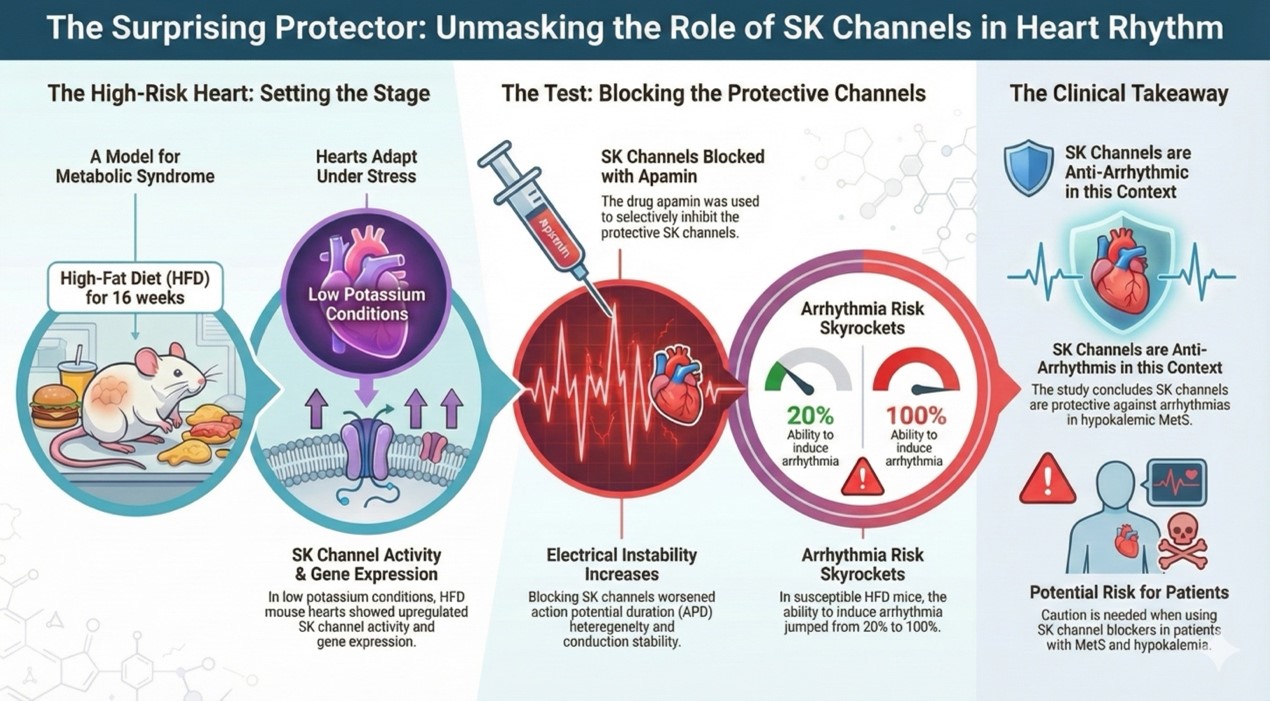
Figure 2. The association between the SK channel and heart rhythm.
Kuo, C.-H., Shih, J.-Y., Lin, Y.-H., Chang, C.-W., Jhuo, S.-J., Lin, T.-C., Huang, T.-C., Liu, I. H., Kao, P.-H., Lin, E.-Y., Huang, P.-C., Chen, X.-H., Chen, C.-Y., Feng, Y.-C., Zheng, Y.-X., Lin, M.-H., Chen, G.-L., Hsu, P.-C., Lee, C.-H., . . . Tsai, W.-C. (2025). Atrial arrhythmogenesis and electrophysiological remodeling in hypokalaemic metabolic syndrome: the role of apamin-sensitive SK channels. EP Europace, 27(8). https://doi.org/10.1093/europace/euaf159
