-Office of Research and Development, KMU -
The Major Challenges of ICB Immunotherapy: Adverse Effects and MHC Limitations
Immune checkpoint inhibitors (ICBs) have revolutionized cancer treatment by blocking inhibitory molecules such as CTLA-4, PD-1, and PD-L1, thereby reactivating the immune system to fight tumors [1–5]. While these therapies have shown promising results in cancers like melanoma and lung cancer, they are not without limitations. One of the most pressing issues is the frequent occurrence of severe immune-related adverse effects. For instance, although the CTLA-4 inhibitor Yervoy (ipilimumab) can effectively stimulate immune responses, it often causes systemic immune hyperactivation, leading to serious side effects such as colitis, hepatitis, endocrinopathies, dermatologic toxicity, and even neuroinflammation [6–9]. These adverse events can necessitate treatment discontinuation and significantly impact patient quality of life and prognosis. Therefore, improving the tumor selectivity of ICBs is an urgent need.
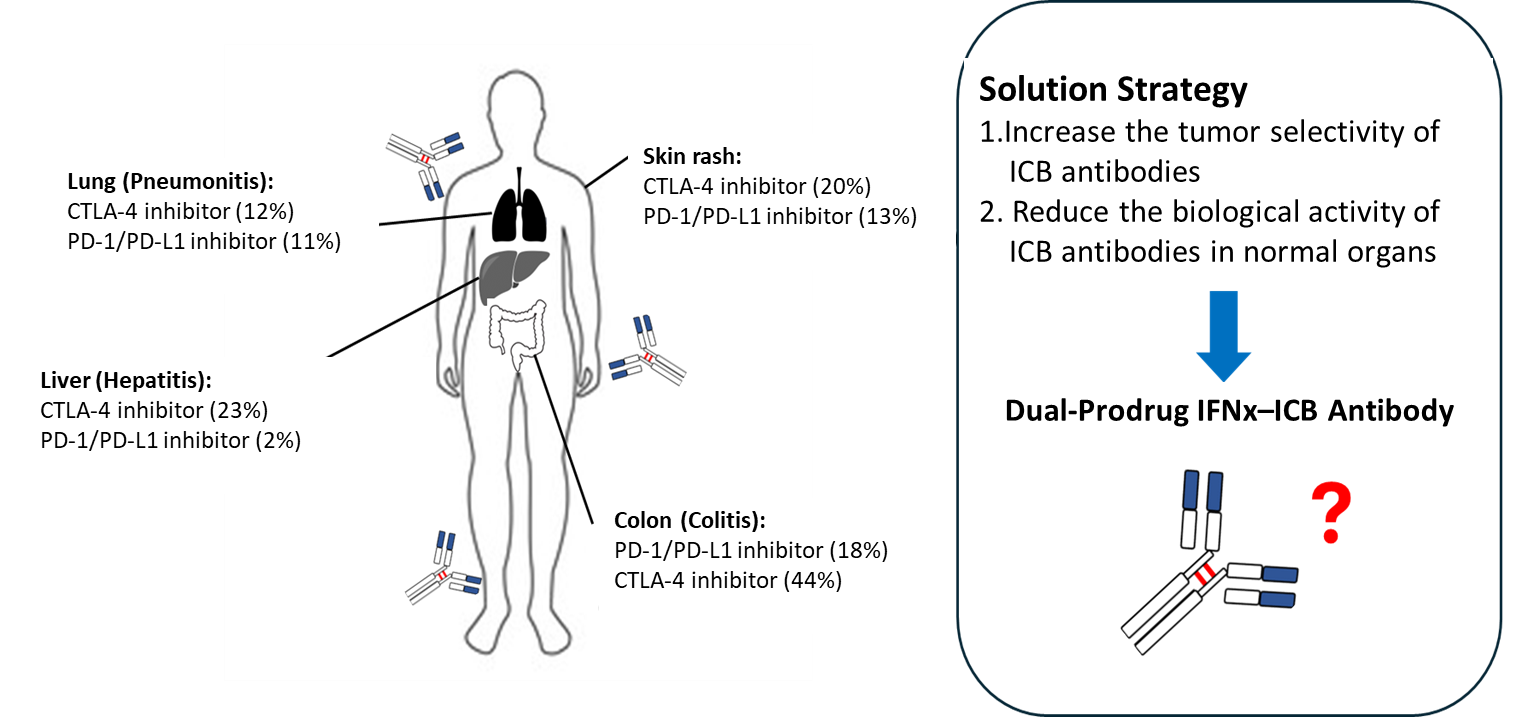
Another key limitation lies in the tumor’s ability to evade immune recognition through downregulation of MHC I (major histocompatibility complex class I), which is essential for T cell-mediated tumor antigen recognition. Many tumors actively reduce MHC I expression, thus escaping immune detection. For example, patients with melanoma lacking beta-2-microglobulin—a critical component of MHC I—show a markedly reduced response to Yervoy (only about one-fourth that of patients with normal MHC I levels) and have poorer outcomes overall. In summary, two major obstacles hinder the clinical effectiveness of ICBs: (1) lack of tissue selectivity resulting in systemic toxicity and (2) insufficient MHC I expression in tumors, limiting immune recognition and therapeutic efficacy. To address these challenges, researchers are actively developing safer, more targeted immunotherapeutic solutions.
Our Solution: A Tumor-Selective IFNγ-Yervoy Fusion Antibody
To overcome these challenges, our team has developed a tumor-selective prodrug fusion antibody, IFNγ–Yervoy, designed to tackle both the safety issues and the immune evasion strategies of tumors. This novel therapeutic incorporates a matrix metalloproteinase-2 (MMP-2) cleavable peptide linker between interferon-gamma (IFNγ) and the CTLA-4 inhibitor Yervoy (ipilimumab). The fusion antibody remains inactive in the bloodstream and normal tissues, avoiding systemic immune activation. However, upon reaching the tumor microenvironment—characterized by high MMP-2/9 activity—the linker is cleaved, locally releasing the active IFNγ and Yervoy components.
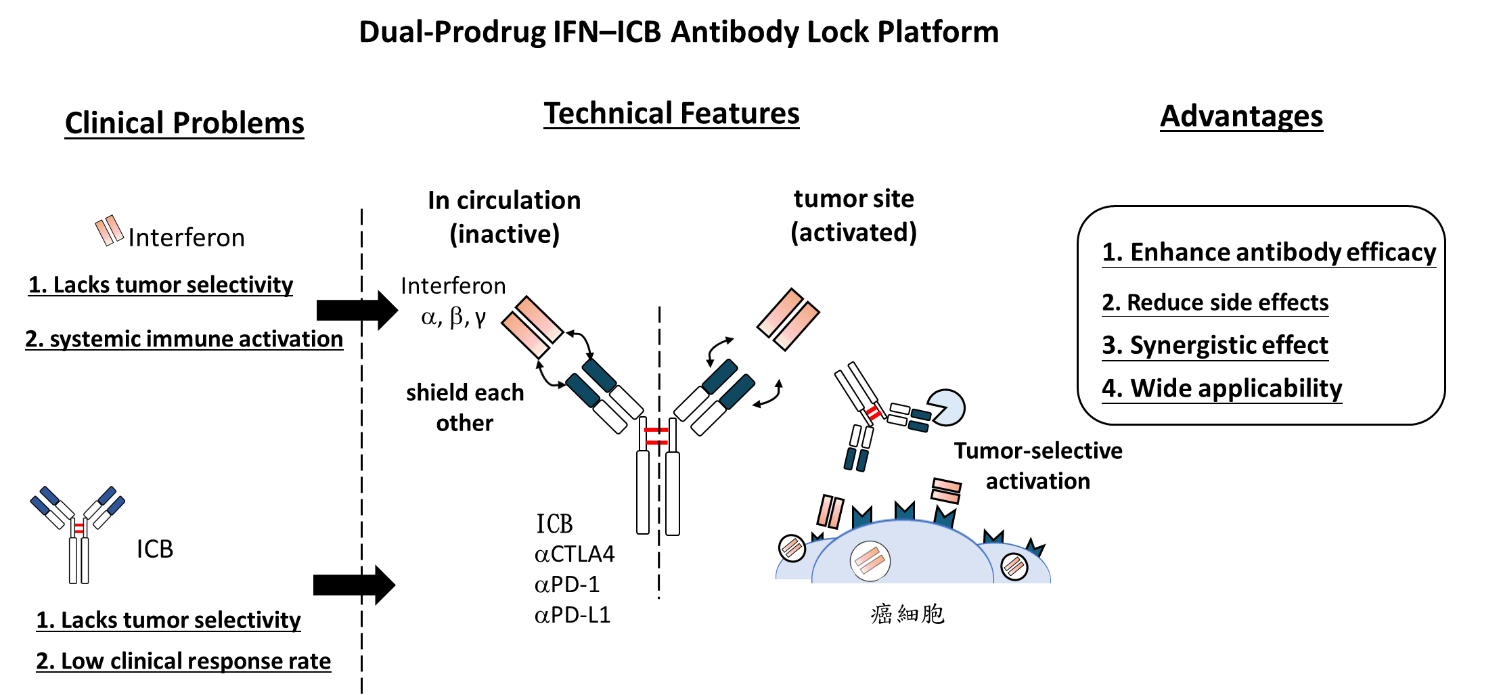
This platform operates like a dual safeguard: Yervoy blocks CTLA-4 to activate T cells, while IFNγ enhances MHC I expression on tumor cells, improving immune system recognition and attack. The synergistic effects not only enhance therapeutic efficacy but also significantly reduce systemic toxicity, achieving a more "tumor-targeted yet normal tissue-friendly" immunotherapy.
Our Innovative Strategy: The Dual-Prodrug IFNx–ICB Antibody Lock Platform
The core of our platform lies in combining interferons (IFNs) with immune checkpoint inhibitors (ICBs) and integrating MMP-2/9-cleavable peptide sequences to enable tumor-selective activation. This dual-prodrug antibody strategy harnesses both the immunostimulatory power of IFNs and the checkpoint-blocking effect of ICBs.
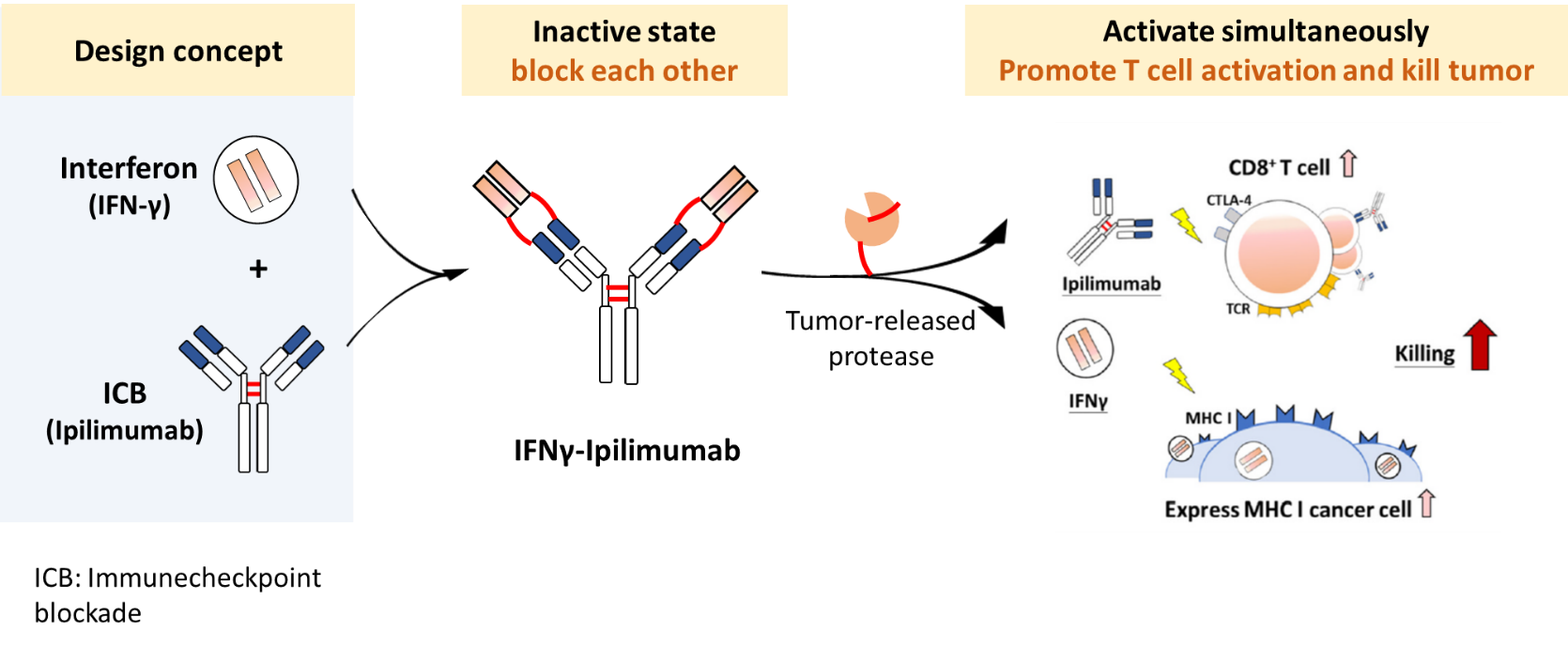
IFNs activate immune effector cells, such as T cells and NK cells, boosting their cytotoxicity toward cancer cells.ICB antibodies (e.g., anti-PD-1, anti-CTLA-4) relieve inhibitory signals that suppress immune responses in the tumor microenvironment. However, when administered systemically, both IFNs and ICBs can trigger severe immune-related side effects. To address this, our design incorporates MMP-2/9-sensitive cleavage sites between IFN and the antibody component. Since MMP-2/9 is highly expressed in the tumor microenvironment, the fusion protein remains inactive in circulation but becomes activated only upon entering the tumor. This mechanism ensures precise spatial control of immune activation, enhancing antitumor efficacy while minimizing systemic toxicity.
Functional Validation and In Vivo Efficacy
In functional assays, MMP-2/9-cleaved IFNγ–Yervoy significantly upregulated MHC I expression in SW480 colorectal cancer cells and markedly enhanced the cytotoxicity of peripheral blood mononuclear cells (PBMCs) across all effector-to-target (E:T) cell ratios tested.
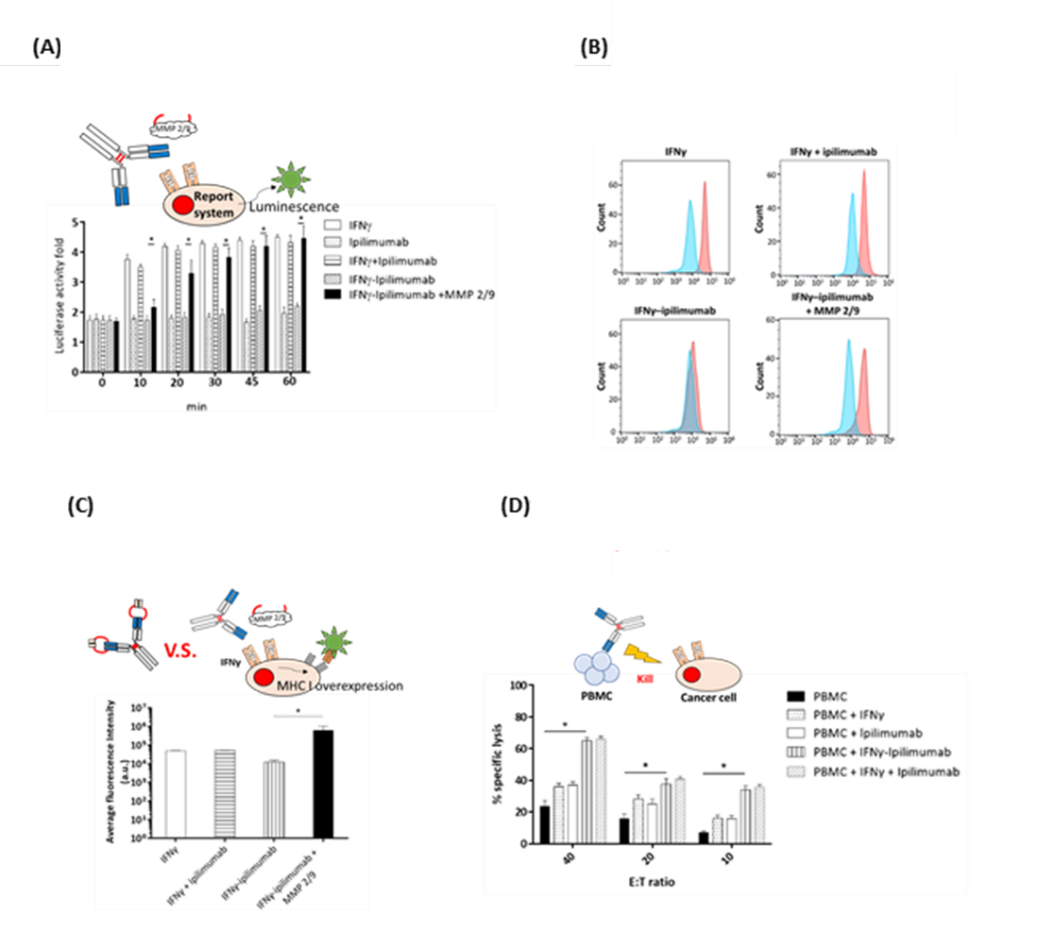
Further in vivo studies using xenograft tumor models demonstrated the therapeutic potential of this fusion antibody. Mice were divided into treatment groups including PBS control, IFNγ alone, Yervoy alone, the combination of both, and the IFNγ–Yervoy fusion antibody. Results showed that IFNγ–Yervoy not only significantly inhibited tumor growth but also increased the infiltration of CD4+ and CD8+ T cells into the tumor. Moreover, MHC I expression in tumor cells was enhanced, and the proportions of intratumoral lymphocytes, including CD4+/Foxp3+, CD8+/Foxp3+, and CD16+ subsets, were significantly elevated—indicating effective reprogramming of the tumor microenvironment to favor immune attack.
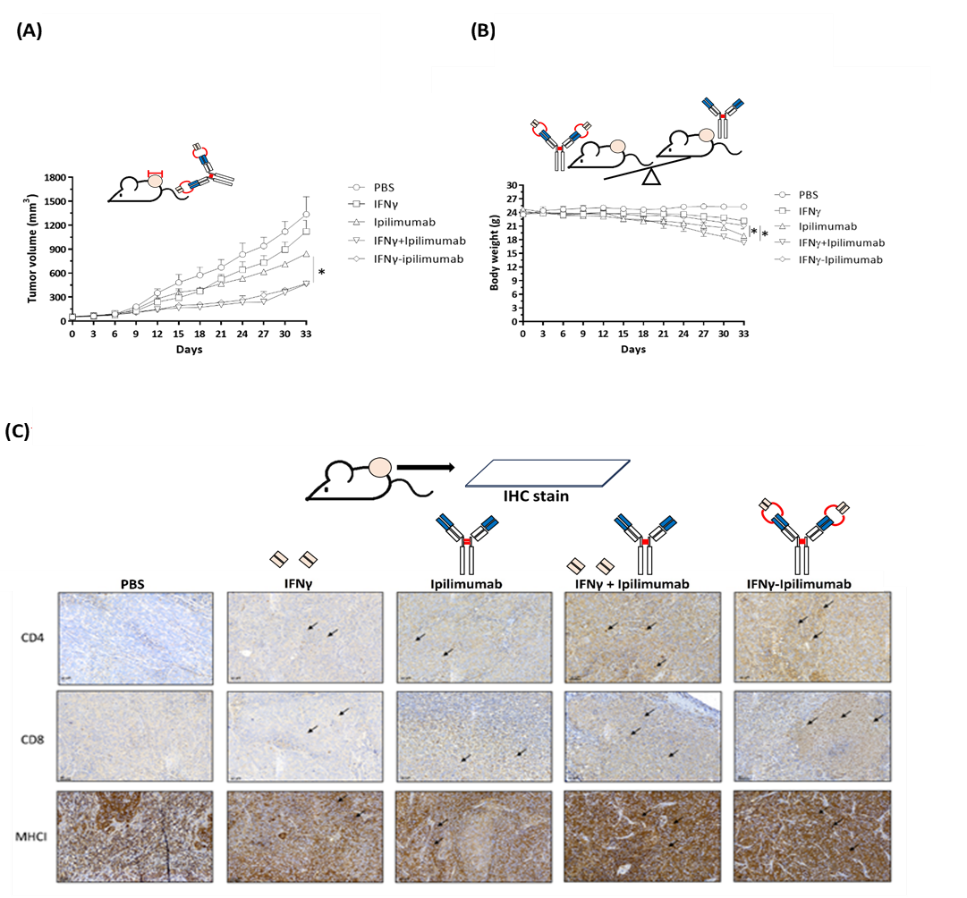
Safety Profile and Therapeutic Promise
Importantly, the IFNγ–Yervoy fusion antibody exhibited excellent safety in vivo. No significant toxicity or organ damage was observed in treated mice, as evidenced by survival rates and liver/kidney function tests (e.g., GOT, GPT, and creatinine levels). These findings highlight the treatment’s favorable safety profile and clinical potential.
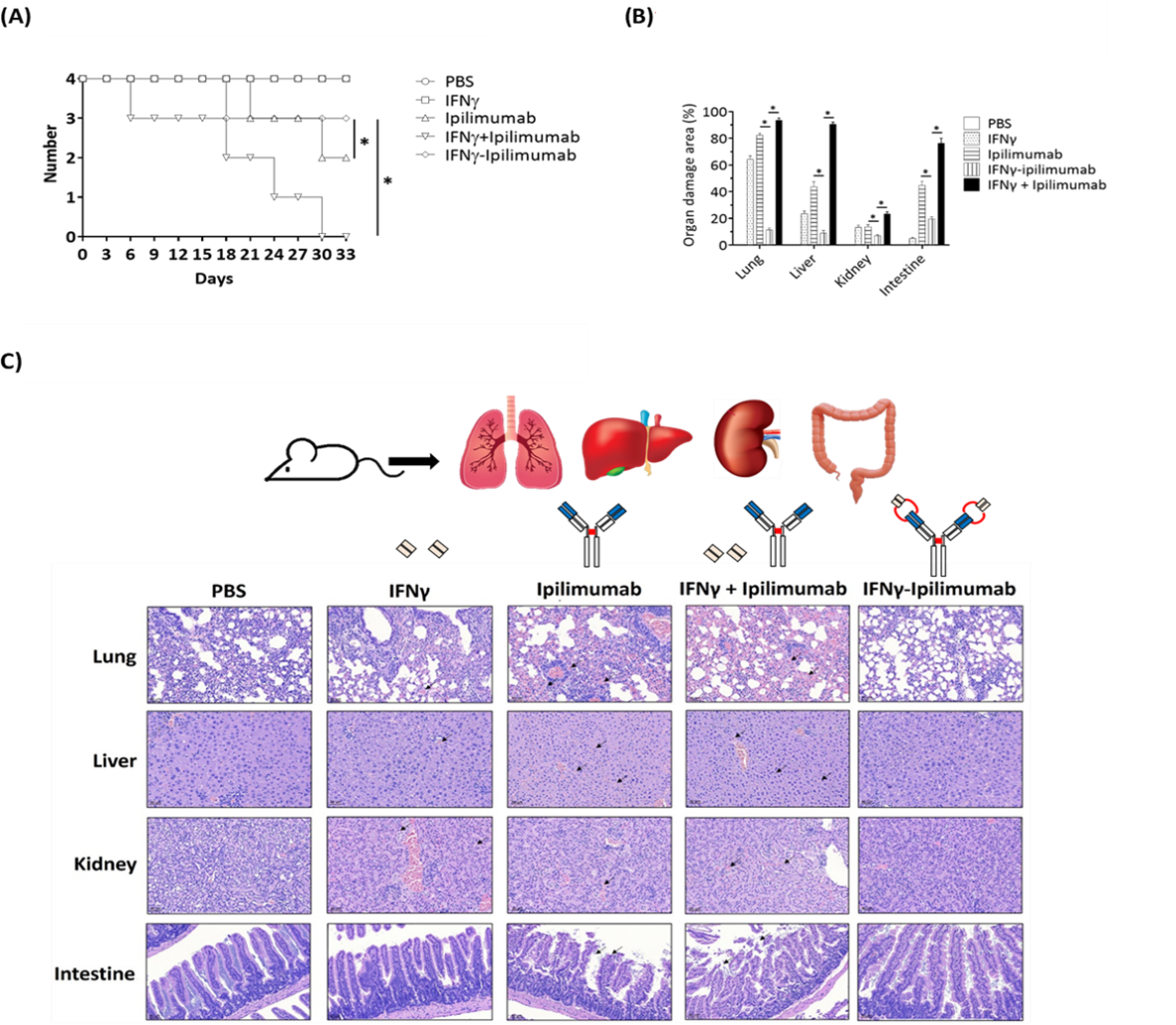
Achievements and Outlook
This research, led by Assoc. Prof. Chih-Hung Chuang and the Center for Drug Development and Value Creation, was published in International Journal of Biological Macromolecules in June 2024 under the title “Selective activation of IFNγ–ipilimumab enhances the therapeutic effect and safety of ipilimumab.” The journal has an impact factor of 8.2 and ranks in the top 8.1% in the "Applied Chemistry" category. The technology has been protected by a U.S. provisional patent application (111028-US-PRO) and a PCT international patent application.
This research was also honored with the 21st National Innovation Award (Academic Research Category) in Taiwan. By demonstrating that selective activation of IFNγ and ipilimumab significantly improves both therapeutic efficacy and safety, this work offers a new direction and a safer alternative for future cancer immunotherapies. Its publication in a high-impact journal underscores its scientific and clinical significance.

研究聯繫Email:a4132600@gmail.com
論文出處:Selective activation of IFNγ-ipilimumab enhances the therapeutic effect and safety of ipilimumab. Int J Biol Macromol. 2024 Apr;265(Pt 2):130945.
